By Chrissy Vitale, DO (a.k.a. Codename – Flygirl)

Yay! The heat has finally broke and it is time to dust off that old sweater in the closet. You go to put it on, and it is riddled with holes from hungry moth larvae. Gross! Sound familiar? Yeah…me neither. But our parents and grandparents may be more familiar with this experience.
Their solution to the problem…MOTHBALLS!
If you are like me, you are probably thinking…What the Heck is a Mothball?
Now your next question should be, “What are mothballs made of?” And I promise I will answer that shortly. But if you are a true Safety Ranger, you know the real question is “If mothballs kill moths, are they toxic to humans?”
The simple answer that the question is “Yes”,but let’s be brave, grab our flashlights and really find out about these little monsters hidden deep in grandma’s closet.
There are 3 Types of Mothballs based on Composition:
Let’s discuss them one-by-one.
Camphor is an essential oil originally distilled from the bark of the camphor tree. The fumes from camphor mothballs can cause symptoms such as headache, nausea, and vomiting in humans. But the real toxicity starts it they are ingested.

Remember, Mothballs May Look Like Candy, But Do Not Eat Them!
If a child or if anyone ingests a camphor mothball, they can experience many symptoms. The first symptoms of camphor toxicity occur quickly (within 5 to 90 minutes), and can include burning of the mouth and throat, nausea, and vomiting. However, infants and young toddlers cannot say, “Mommy my mouth hurts”. Most ingestions resulting in trips to the hospital are due to the neurological affects, most commonly seizures, in addition to confusion, muscular contractions, vision problems and possible death. Fortunately, in 1980, the FDA banned the nonprescription sale of camphorated oil due to accidental ingestion. In 1983, the FDA put a restriction on camphor content to less than 11% in nonprescription camphor containing products in the United States. So camphor can still be found in some products, however, if you want camphor mothballs, you have to take a trip to China.
But instead of traveling overseas, let us travel back in time to discuss our next mothball. In the 1800s, chemists were experimenting with purified coal tar and its odorous properties. With further research, naphthalene was discovered and later the naphthalene mothball was created. Now let me tell ya, naphthalene is not much of an improvement from camphor. The fumes from naphthalene can also cause headaches, nausea, dizziness, and vomiting, but if someone breathes in enough of the vapor or even eats a mothball, that’s when things get serious.
Safety Rangers…Congratulations! We have fast-forwarded in time and you are now emergency medicine physicians! It is a typical day in the department, and it is time to see your next patient. You walk into the room and see a concerned mother with two infants. Immediately you notice something different about the children, but they seem to be in no distress. You get the history from the mother who says that the kids spent the weekend at their grandparent’s house. When the mother went to pick them up, they looked like this:
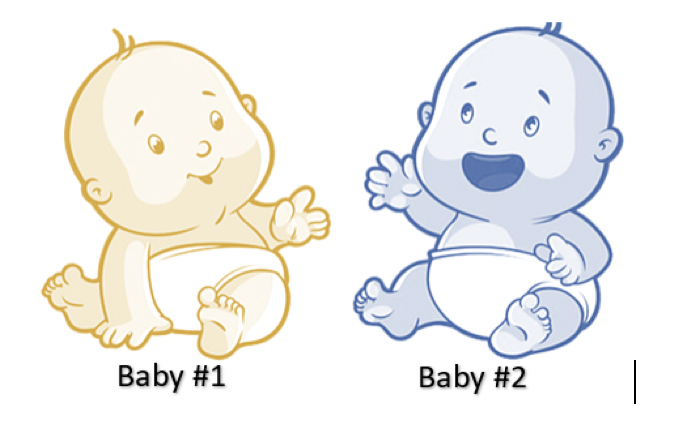
You examine the kiddos and still being a Safety Ranger, you notice a familiar nostalgic smell on their clothing. You ask if the mother’s parents use mothballs in their house. The mother says, “yes”.
What is going on?!
Why is one baby yellow and why is the other baby blue?
Did they both eat a naphthalene mothball?
What is the mechanism for this to happen?
Okay okay…you got me. Just for the record, this is a completely fabricated scenario just for educational purposes. I mean, it could possibly happen, but I digress.
Naphthalene can cause something called hemolytic anemia. Heme means blood and –lytic means breaks. Naphthalene causes your red blood cells to rupture leading to a decrease in the number of red blood cells, also known as anemia. Signs and symptoms of hemolytic anemia include pale skin, yellow skin/eyes like Baby #1 above, dark-colored urine, fever, weakness, dizziness, and increased heart rate.
Not only that, naphthalene can also cause…wait for it…methemoglobinemia. Yeah…I know. Bear with me. To explain this one, we have to go microscopic. For simplicity, blood is made of red blood cells. Within those cells, there is a protein called hemoglobin. This protein is responsible for carrying oxygen throughout the body. The binding sites for oxygen is iron and it normally exist in the ferrous state (Fe2+). Naphthalene changes iron to the ferric state (Fe3+). Unfortunately, hemoglobin in this state is unable to bind oxygen. This molecule is called a methemoglobin. When this molecule accumulates in the body, the condition is called methemoglobinemia. Since the blood does not contain as much oxygen, the blood becomes a chocolate brown color. Signs and symptoms of methemoglobinemia include gray-blue skin discoloration like Baby #2 above, headache, nausea, fatigue, seizures, metabolic acidosis and even death.

Wait! There is more! Naphthalene is very lipophilic, or fat loving, meaning it can be stored in adipose tissue and your brain. This can result in agitation, seizures, coma or even death! And to top it off, the World Health Organization (WHO) concluded that naphthalene is possibly carcinogenic to humans based on animal studies. Fortunately, the production of naphthalene mothballs has decreased since the production of our next mothball. Put do not let your guard down. Naphthalene still lurks on the shelves of certain convenience stores. BEWARE!
And last we get to explore the para-dichlorobenzene mothballs. Hang in there with me Safety Rangers. We are almost done. Para-dichlorobenzene was first registered for use in the United States by the United States Department of Agriculture (USDA) in 1947. It greatly replaced the naphthalene mothball in the second half of the 19th century. Para-dichlorobenzene is said to be the least toxic of the three, but it is not toxic-free. People who are exposed to its vapors may experience nausea, vomiting, dizziness, fatigue, and headaches. It is also an irritant to oral mucosa, nasal mucosa and can cause a burning sensation when it contacts skin. Fortunately, if ingested, it is relatively harmless to humans, but not so much to animals. If a pet eats a mothball made of paradichlorobenzene, they may have vomiting, tremors, abdominal pain and may also cause kidney and liver damage in pets. And the WHO considers paradichlorobenzene possibly carcinogenic to humans based on studies with mice. Even though paradichlorobenzene mothballs are “less toxic”, I still do not want them in my house.
And there you have it Safety Rangers. The journey through different mothballs and their toxicities has come to an end. Unlike some ingestions or exposures, mothballs of any composition do not have a clear antidote. Fortunately, clothing care has evolved over time to how we clean, preserve, and protect our garments. And with innovations such as central heat and air conditioning, synthetic garment materials and home pest control, moths do not have a chance against your favorite sweater. But if you still feel the need to use mothballs, always follow label instructions and take steps to avoid exposure. If any exposures occur, be sure to follow the First Aid instructions on the product label carefully. For additional treatment advice, contact the Poison Control Center at 1-800-222-1222.
If you are still curious about mothballs, head on over to the Poisonboy Podcast where Poison Boy himself, the Copper Kid and myself talk not only about mothballs, but the mysterious case of Mothboy.
References
“Camphor: Uses, Side Effects, Interactions, Dosage, and Warning.” WebMD, WebMD, www.webmd.com/vitamins/ai/ingredientmono-709/camphor.
Center for Drug Evaluation and Research. “Rulemaking History for OTC Camphorated Oil Drug Products.” U.S. Food and Drug Administration, FDA, 1 Jan. 2020, www.fda.gov/drugs/status-otc-rulemakings/rulemaking-history-otc-camphorated-oil-drug-products.
Farkas, Josh. “Methemoglobinemia.” EMCrit Project, 18 June 2020, emcrit.org/ibcc/methemoglobinemia/.
Galbo, Mark J. “Naphthalene and Paradichlorobenzene.” AccessMedicine, accessmedicine.mhmedical.com/content.aspx?bookid=391.
Gervais, J.; Luukinen, B.; Buhl, K.; Stone, D. 2010. Naphthalene General Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. http://npic.orst.edu/factsheets/naphgen.html.
Gervais, J.; Luukinen, B.; Buhl, K.; Stone, D. 2010. Paradichlorobenzene General Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. http://npic.orst.edu/factsheets/PDBgen.html.
Gervais, J.; Luukinen, B.; Buhl, K.; Stone, D. 2010. Paradichlorobenzene Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. http://npic.orst.edu/factsheets/archive/PDBtech.html.
“Hemolytic Anemia.” Hemolytic Anemia | Johns Hopkins Medicine, 2020, www.hopkinsmedicine.org/health/conditions-and-diseases/hemolytic-anemia.
Larasati, Ayu. “Mothballs: Why You Don’t Actually Need It.” Medium, Treeusable 2018, 13 July 2018, medium.com/treeusable/mothballs-why-you-dont-actually-need-it-and-how-to-still-protect-your-clothes-72913572f56c.
“Mothballs: The Familiar Friendly Devil.” BuzzOnEarth, 8 Apr. 2020, www.buzzonearth.com/mothballs-the-familiar-friendly-devil/.